- 1. &
ADSORPTION
By SYEDA MARYAM HASSNY
- 2. DEFINITION
ABSORPTION ADSORPTION
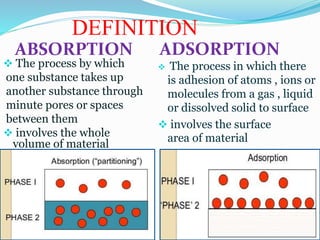
The process by which
one substance takes up
another substance through
minute pores or spaces
between them
involves the whole
volume of material
The process in which there
is adhesion of atoms , ions or
molecules from a gas , liquid
or dissolved solid to surface
involves the surface
area of material
- 4. ADSORBATE
The substance that
gets adsorbed on to
the surface
ADSORBENT
The substance on the
surface of which
adsorption takes
place
EXAMPLE
If a gas is adsorbed
onto the surface of
the solid , then the
gas is termed as
ADSORBATE and
the solid is termed as
ADSORBENT
- 5. ABSORBENT
A material having
capacity or
tendency to
absorb another
substance
ABSORBATE
A material that
has been or is
capable of being
absorbed
ABSORBENT
ABSORBATE
ABSORBENT ABSORBATE
- 6. ADSORPTION - Vs - ABSORPTION
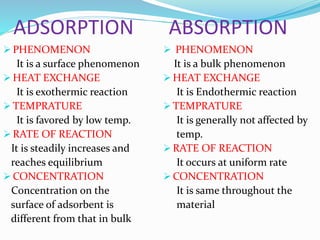
- 7. ADSORPTION ABSORPTION
PHENOMENON
It is a surface phenomenon
HEAT EXCHANGE
It is exothermic reaction
TEMPRATURE
It is favored by low temp.
RATE OF REACTION
It is steadily increases and
reaches equilibrium
CONCENTRATION
Concentration on the
surface of adsorbent is
different from that in bulk
PHENOMENON
It is a bulk phenomenon
HEAT EXCHANGE
It is Endothermic reaction
TEMPRATURE
It is generally not affected by
temp.
RATE OF REACTION
It occurs at uniform rate
CONCENTRATION
It is same throughout the
material
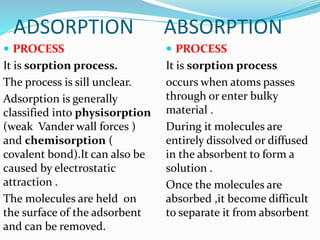
- 8. ADSORPTION ABSORPTION
PROCESS
It is sorption process.
The process is sill unclear.
Adsorption is generally
classified into physisorption
(weak Vander wall forces )
and chemisorption (
covalent bond).It can also be
caused by electrostatic
attraction .
The molecules are held on
the surface of the adsorbent
and can be removed.
PROCESS
It is sorption process
occurs when atoms passes
through or enter bulky
material .
During it molecules are
entirely dissolved or diffused
in the absorbent to form a
solution .
Once the molecules are
absorbed ,it become difficult
to separate it from absorbent
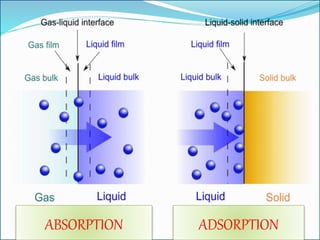
- 9. ABSORPTION ADSORPTION
- 10. TYPES OF ADSORPTION
PHYSICAL
ADSORPTION
CHEMICAL
ADSORPTION
TYPES OF ABSORPTION
PHYSICAL
ABSORPTION
CHEMICAL
ABSORPTION
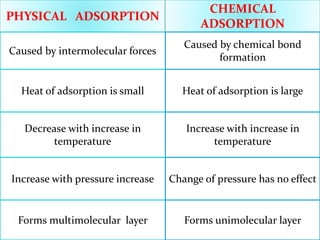
- 11. PHYSICAL ADSORPTION
CHEMICAL
ADSORPTION
Caused by intermolecular forces
Caused by chemical bond
formation
Heat of adsorption is small Heat of adsorption is large
Decrease with increase in
temperature
Increase with pressure increase
Forms multimolecular layer
Increase with increase in
temperature
Change of pressure has no effect
Forms unimolecular layer
- 13. CHEMICAL PHYSICAL
ABSORPTION ABSORPTION
Chemical
absorption is a
chemical reaction
between absorbed
and the absorbing
substance
Physical absorption
is in which the
absorbent and
absorbate develops
weak Vander wall
force and it can be
reversible
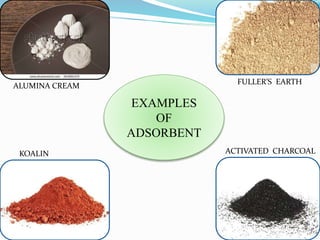
- 15. EXAMPLES
OF
ADSORBENT
KOALIN ACTIVATED CHARCOAL
ALUMINA CREAM
FULLER’S EARTH
- 16. USES OF ADSORPTION
In TREATMENT of
DIARRHEA
In GAS MASK in adsorbing
ABNOXIOUS GASES
In CLARIFICATION of
SUGAR
In PAINT INDUSTRY
In adsorption of EXCESSIVE
GAS in the
GASTROINTESTINAL
TRACT and BACTERIAL
TOXIN
- 17. EXAMPLES
OF
ABSORBENT
COTTON
GRANULAR
INDUSTRIAL ABSORBENT
ABSORBENT PULP
ABSORBENT POLYMER
- 18. USES OF ABSORPTION
Used in ABSORPTION
CHILLERS for SPACE
COOLING APPLICATION
Use in CLEAN BURNING of
FUELS
The process of gas absorption
by a liquid is used in
HYDROGENATION OF OILS
Used in CARBONATION